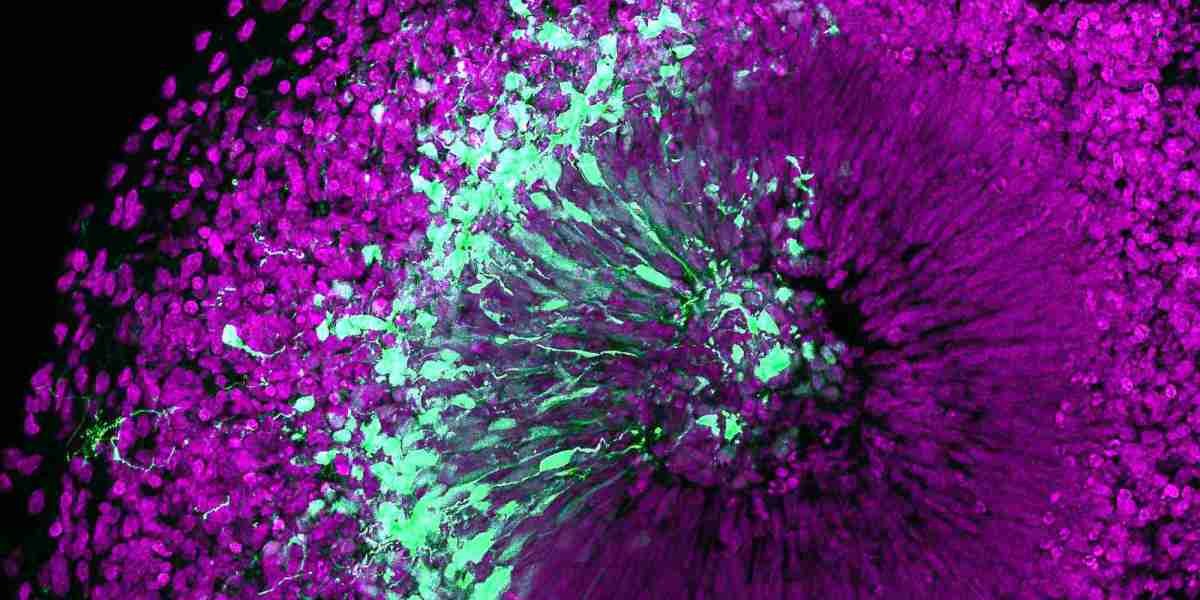
The organoids market, driven by the rapid advancement of biotechnology and tissue engineering, holds transformative potential for medical research, drug development, and personalized medicine. However, despite the promise of these miniature, three-dimensional models of human organs, the market faces several significant barriers that hinder its growth and widespread adoption. These challenges stem from technical, regulatory, financial, and ethical concerns that need to be addressed for the organoids market to reach its full potential.
Technical Limitations
One of the primary barriers to the widespread use of organoids is the technical complexity involved in their development and application. Organoids are intricate cellular structures that replicate aspects of human organs in a lab environment, yet producing them with sufficient accuracy, consistency, and functionality remains a considerable challenge. The current methods for generating organoids often result in variations between batches, leading to reproducibility issues that can undermine their reliability as research tools. Achieving a high degree of maturity and stability in organoids that mirrors actual human organs is also an ongoing struggle. Researchers face limitations in replicating the complex microenvironments and interactions found in human tissues, which is crucial for ensuring the accuracy of data derived from these models.
Scaling organoid production for commercial use presents another significant hurdle. While organoids show immense potential for personalized medicine, scaling them up for widespread application in drug discovery and therapeutic interventions requires overcoming substantial obstacles in terms of cost, labor, and technical capacity. Achieving mass production without compromising quality is critical to making organoids viable for large-scale adoption in pharmaceutical and healthcare industries.
Regulatory and Ethical Challenges
Another critical barrier to the growth of the organoids market is the complex and evolving regulatory landscape. As organoids are derived from human stem cells, they raise a host of ethical and regulatory concerns, particularly around sourcing and the use of human tissue. Regulatory agencies such as the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency) have yet to establish standardized guidelines for the approval and commercialization of organoid-based therapies. Without clear regulatory frameworks, researchers and companies may hesitate to invest in organoid technologies, further delaying their integration into clinical settings.
Ethical concerns also revolve around the potential for organoids to be used in ways that challenge societal norms and safety regulations. The risk of creating human-like models that could potentially evolve into more complex organisms has raised questions within the scientific and public communities about the boundaries of such research. Ensuring that ethical practices are strictly followed will be essential in maintaining public confidence and ensuring regulatory compliance. Additionally, the global diversity of regulations makes international collaboration more difficult and adds a layer of complexity to the commercialization process for organoid-based products.
Cost and Financial Constraints
The development and production of organoids require substantial financial investment. High research and development (R&D) costs, coupled with the need for advanced facilities and expertise, create financial barriers for companies and research institutions. Many early-stage companies focusing on organoid technology are still in the discovery phase, making it difficult to attract investment from venture capitalists who often seek quicker returns on their investments. The high cost of research and the uncertainty surrounding the commercial viability of organoid-based products have led to cautious investment strategies in the industry.
Organoid technology has not yet reached the stage where it can be routinely integrated into pharmaceutical pipelines or clinical practices. Until organoids are proven to deliver reliable and reproducible results, companies may be hesitant to allocate significant financial resources toward their development. This limits the ability to scale organoid research into profitable commercial ventures.
Market Awareness and Education
Another barrier to the growth of the organoids market is the lack of widespread awareness and education surrounding their potential. While organoid research is gaining momentum in academic and biotechnology circles, the broader healthcare industry, including clinicians, pharmaceutical companies, and regulatory bodies, may not fully understand the scope and benefits of this technology. This knowledge gap creates hesitation among stakeholders who are unsure about integrating organoids into their workflows.
Increasing market awareness and educating key players in the industry about the potential benefits of organoid technology could go a long way in alleviating some of these barriers. For example, organoids can provide better preclinical models for drug testing, enabling more accurate predictions of human responses to pharmaceuticals. Overcoming these educational barriers will be critical to speeding up the adoption of organoid-based research tools in both drug discovery and clinical applications.