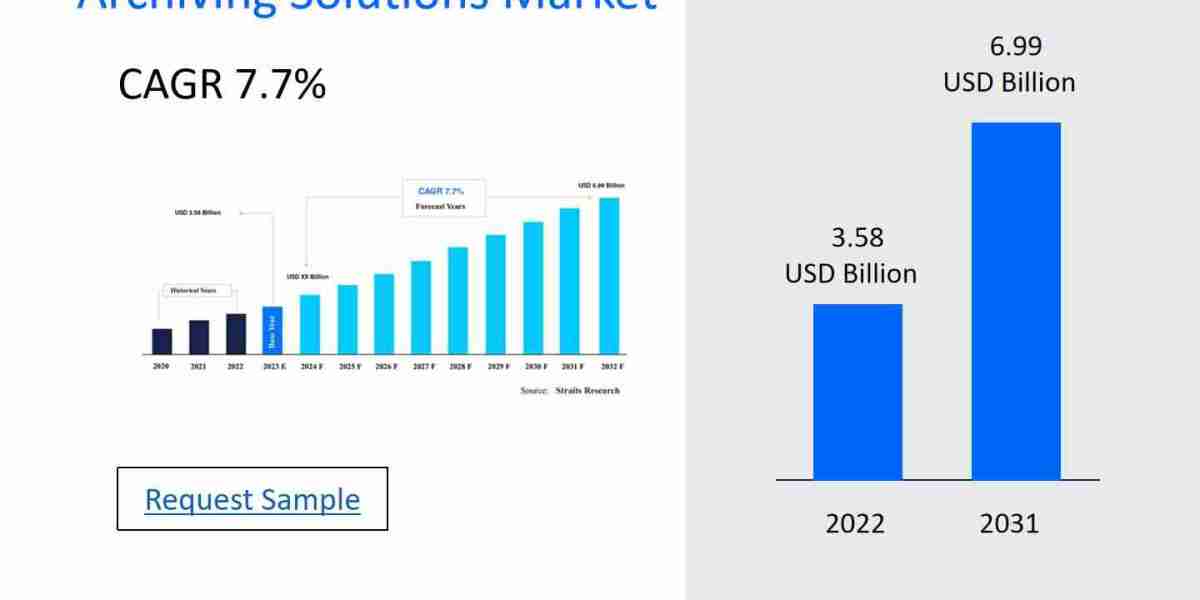
Clinical Trial Biorespository Archiving Solutions Market
The Clinical Trial Biorespository Archiving Solutions Market includes a broad array of products, services, and systems aimed at enhancing individuals' well-being. It covers everything from healthcare services in clinics and hospitals to pharmaceuticals, medical devices, insurance, and digital health solutions. Factors such as an aging population, advancements in medical technology, and increasing demand for personalized care drive the market's rapid expansion and evolution. The sector features a diverse range of key players, from conventional healthcare providers to tech companies venturing into telemedicine and health data analytics. This industry faces challenges including regulatory requirements, elevated costs, and the necessity for accessible and equitable care, making innovation and adaptation crucial.
Get Free Sample Report PDF @ https://straitsresearch.com/report/clinical-trial-biorespository-archiving-solutions-market/request-sample
Economically, the Clinical Trial Biorespository Archiving Solutions Market constitutes a significant share of GDP in numerous developed nations, while also exhibiting consistent growth in emerging markets. The sector comprises a combination of public and private organizations, all of which enhance the infrastructure for delivering healthcare services. Various stakeholders, including insurance companies, pharmaceutical producers, healthcare IT firms, and service providers, contribute to a competitive landscape where patient requirements, efficiency, and cost-effectiveness are major drivers of innovation.
Some of the key players profiled in the study are
- Medpace
- Atcc
- CellandCo Bioservices
- Brooks Life Sciences
- Patheon (Thermo Fisher Scientific Inc.)
- Precision Medicine Group LLCs.
- Labcorp Drug Development
- Q2 Solutions
- Charles River Laboratories
Scope of Report on the Clinical Trial Biorespository Archiving Solutions Market
This document delivers an in-depth examination of the global Clinical Trial Biorespository Archiving Solutions Market, providing insights into important segments, prevailing trends, factors driving growth, obstacles, and the competitive environment influencing the sector. The Clinical Trial Biorespository Archiving Solutions Market, a broad field encompassing pharmaceuticals, medical devices, healthcare services, biotechnology, and health IT industries, is analyzed from various perspectives to offer a comprehensive view of both existing circumstances and future directions.
The Report is Segmented as Follows
- By Service
- Biorepository Services
- Archiving Solution Services
- By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
- By Products
- Preclinical Products
- Clinical Products
Get Detailed Segmentation @ https://straitsresearch.com/report/clinical-trial-biorespository-archiving-solutions-market/segmentation
Objective of Report on the Clinical Trial Biorespository Archiving Solutions Market
The aim of this report is to present a thorough analysis of the existing landscape, trends, and key factors influencing the Clinical Trial Biorespository Archiving Solutions Market. This report seeks to provide insights into the structure of the market, its growth potential, and the elements affecting healthcare service delivery, expenses, and global accessibility. By analyzing different segments—including pharmaceuticals, medical devices, healthcare services, and digital health technologies—the report aims to underscore the strengths, weaknesses, opportunities, and challenges facing the industry.
Additionally, the report plans to assess how demographic changes, technological progress, regulatory shifts, and socioeconomic factors are shaping healthcare demand and provision. Through an in-depth exploration of emerging trends and innovations, we intend to deliver practical insights for stakeholders, including healthcare providers, investors, policymakers, and industry professionals, to assist in making informed decisions and strategic planning within the healthcare ecosystem.
Key Questions Answered in the Clinical Trial Biorespository Archiving Solutions Market Analysis
- What are the main factors contributing to growth in the Clinical Trial Biorespository Archiving Solutions Market?
- How are evolving regulatory frameworks affecting Clinical Trial Biorespository Archiving Solutions Market providers and their stakeholders?
- What are the key obstacles confronting Clinical Trial Biorespository Archiving Solutions Market in attaining sustainable growth?
- How are changes in consumer expectations within Clinical Trial Biorespository Archiving Solutions Market influencing service delivery?
- What are the new trends in financing and reimbursement within Clinical Trial Biorespository Archiving Solutions Market?
Buy Clinical Trial Biorespository Archiving Solutions Market Research Report @ https://straitsresearch.com/buy-now/clinical-trial-biorespository-archiving-solutions-market
Region Included are
Global, North America, Europe, APAC, South America, Middle East & Africa, LATAM.
Country Level Break-Up: United States, Canada, Mexico, Brazil, Argentina, Colombia, Chile, South Africa, Nigeria, Tunisia, Morocco, Germany, United Kingdom (UK), the Netherlands, Spain, Italy, Belgium, Austria, Turkey, Russia, France, Poland, Israel, United Arab Emirates, Qatar, Saudi Arabia, China, Japan, Taiwan, South Korea, Singapore, India, Australia and New Zealand etc.
At long last, Clinical Trial Biorespository Archiving Solutions Market is an important source of direction for people and companies.
The market research report on the Global Clinical Trial Biorespository Archiving Solutions Market has been thoughtfully compiled by examining a range of factors that influence its growth, including environmental, economic, social, technological, and political conditions across different regions. A detailed analysis of data related to revenue, production, and manufacturers provides a comprehensive view of the global landscape of the Clinical Trial Biorespository Archiving Solutions Market. This information will be beneficial for both seasoned businesses and new entrants, assisting them in evaluating the investment prospects in this expanding market.
Thank you for taking the time to read this article; you can also obtain region-specific report versions, including Global, North America, Europe, APAC, South America, Middle East & Africa, and LAMEA) along with Forecasts for 2024-2032.
About US
Regardless of whether you're looking at business sectors in the next town or crosswise over continents, we understand the significance of being acquainted with what customers purchase. We overcome the issues of our customers by recognizing and deciphering just the target group, while simultaneously generating leads with the highest precision. We seek to collaborate with our customers to deliver a broad spectrum of results through a blend of market and business research approaches.
Contact US
Email ID : sales@straitsresearch.com
Phone No. : +1 646 905 0080 (U.S.) or +44 203 695 0070 (U.K.)
For More Report :
https://straitsresearch.com/report/minimally-invasive-cosmetic-procedure-market
https://straitsresearch.com/report/gastrointestinal-endoscopy-market
https://straitsresearch.com/report/healthcare-predictive-analytics-market
https://straitsresearch.com/report/idiopathic-pulmonary-fibrosis-market