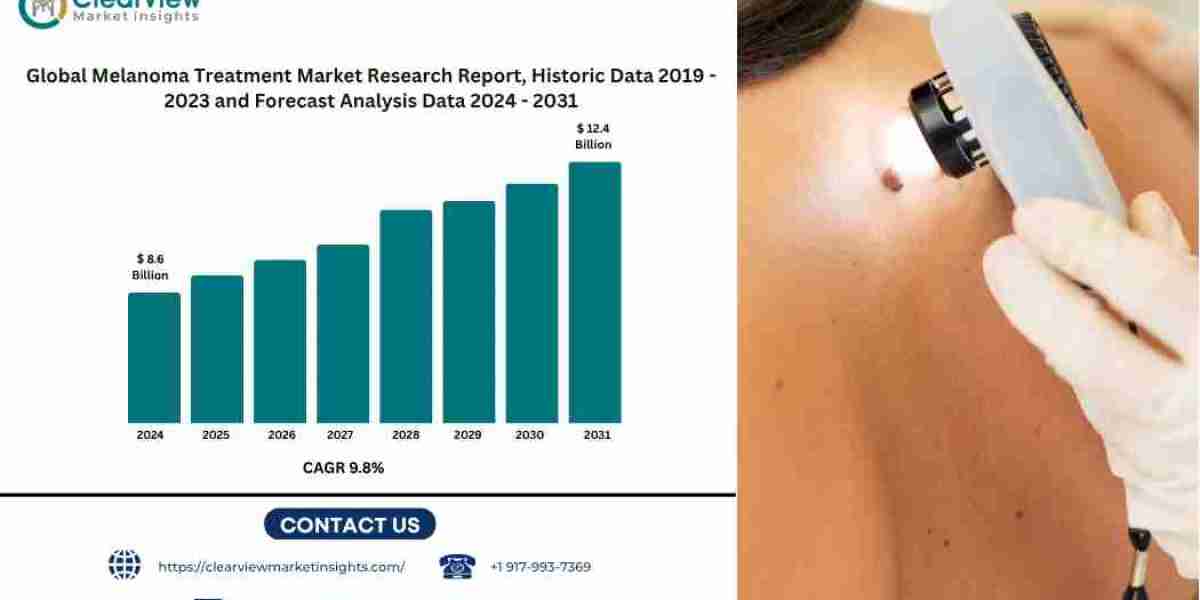
[New York, 17th June 2025] – The global melanoma treatment market is poised for transformative growth, projected to expand from USD 5.2 billion in 2023 to USD 12.4 billion by 2035, at a CAGR of 10.8%, according to Clearview Market Insights. This growth is driven by revolutionary advancements in immune checkpoint inhibitors, targeted therapies, and AI-driven diagnostics, reshaping outcomes for late-stage melanoma patients.
Request Sample @ https://clearviewmarketinsights.com/report-details/global-melanoma-treatment-market/
Key Growth Catalysts
- Immunotherapy Dominance:
- PD-1 inhibitors (Keytruda, Opdivo) now account for 58% of revenue in 2024, with 5-year survival rates doubling to 40% for metastatic melanoma.
- Pipeline Boom: Over 120 clinical trials are underway for next-generation CTLA-4/PD-L1 combinations (e.g., BioNTech’s mRNA-based personalized vaccines).
- Precision Medicine Leap:
- BRAF/MEK inhibitors (e.g., Tafinlar + Mekinist) account for 32% of targeted therapy sales, while biomarker testing adoption has increased to 65% in the U.S. and EU.
- Liquid biopsiesgain traction, reducing treatment resistance risks (market for melanoma liquid assays to hit USD 1.8B by 2030).
- Early Detection Tech:
- AI-powered dermatoscopes (e.g., DermEngine) reduce diagnostic delays by 30% and achieve 90% accuracy in detecting Stage 0–I melanomas.
Competitive Intensity Heats Up
- Merck & Co. (35% share): Strengthens Keytruda’s dominance with new adjuvant approvals for Stage IIB/C melanoma (2024).
- Bristol-Myers Squibb (28%): Investing in the Opdivo+Yervoy combination, which is currently in Phase III trials for rare ocular melanoma.
- Startups Disrupt: Companies like NeraCare (Germany) are pioneering hyperspectral imaging for non-invasive staging.
Regional Front-Runners
- North America (48% share): U.S. leads with 90% insurance coveragefor immunotherapy.
- Europe (XX%): Germany’s universal biomarker testing policy drives 15% annual growth.
- Asia-Pacific (XX%): Japan’s fast-track approvals for novel therapies (e.g., Delcath’s CHEMOSAT) fuel demand.
Patient Access Challenges
Despite progress, cost barriers persist:
- Immunotherapy costs USD 150,000 per year, limiting uptake in emerging markets.
- Only 25% of African nations include melanoma drugs in their national formularies, compared to 85% in the EU.
Future Milestones
- 2026: First FDA-approved mRNA melanoma vaccine(Moderna/BioNTech).
- 2028: AI platforms integrate with EHRs for real-time treatment adjustments.
- 2030: 90% of Stage III patientsreceive combo therapies as standard care.
For more insights, visit https://clearviewmarketinsights.com/
About Clearview Market Insights:
Clearview Market Insights is a leading market research and consulting firm providing in-depth industry analysis and strategic recommendations for businesses worldwide.
Media contact:
Bhavani K
Marketing and Sales Head
ClearView Market Insights
Mail: sales@clearviewmarketinsights.com
Phone: +1 917-993-7369