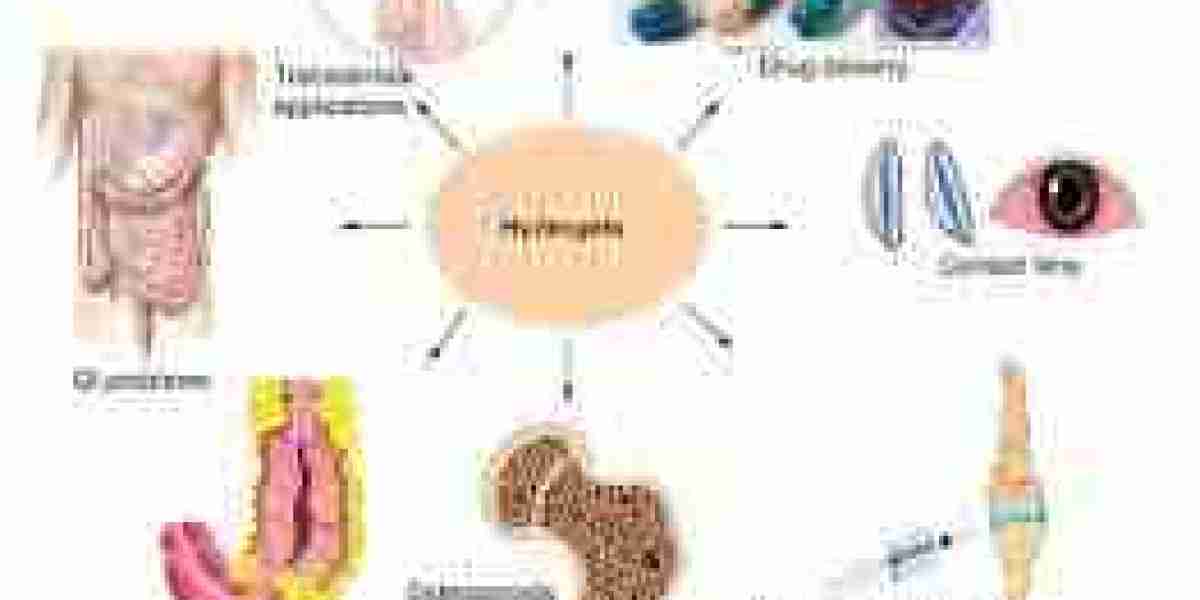
The current scenario of the hydrogel-based drug delivery market is characterized by an accelerating shift from conventional drug administration methods toward advanced biomaterial-based systems that offer improved control, precision, and biocompatibility. This evolving landscape reflects not only technological progress but also a broader transformation in how pharmaceutical therapies are developed, delivered, and evaluated in clinical settings. As hydrogel systems gain traction across multiple therapeutic domains, the market scenario reveals both promising developments and critical strategic considerations.
Presently, the market is in a growth phase marked by increasing adoption of hydrogel-based platforms in various clinical applications. From ophthalmic gels and transdermal patches to injectable depots and implantable scaffolds, hydrogels are being embraced for their versatility and ability to improve patient compliance. Their swelling capacity, tunable degradation, and responsiveness to environmental triggers make them particularly suitable for conditions requiring localized or sustained drug release. Therapeutic areas such as oncology, wound healing, orthopedics, and cardiovascular treatment are witnessing growing incorporation of hydrogel formulations in both commercial and investigational products.
At the same time, the global pharmaceutical industry is increasingly recognizing hydrogels as a valuable enabler of innovation. Several recent product approvals and pipeline advancements feature hydrogel-based delivery systems, signaling a clear trend toward regulatory acceptance. This shift is being supported by more mature material science, improved manufacturing technologies, and refined clinical trial designs that can demonstrate the unique advantages of hydrogel-mediated therapies.
The competitive landscape reflects a balanced mix of established players and emerging innovators. Large pharmaceutical companies are pursuing partnerships with startups and academic labs to access hydrogel technologies that can differentiate their product portfolios. Meanwhile, smaller biotech firms are leveraging specialized expertise to develop proprietary hydrogel systems tailored for specific indications. This collaboration-driven environment is fueling knowledge exchange, accelerating development timelines, and enhancing the overall credibility of hydrogel-based approaches in the eyes of clinicians and regulators.
Regionally, North America continues to lead the market due to its strong research infrastructure, well-established pharmaceutical sector, and favorable reimbursement environment. The U.S., in particular, benefits from the FDA’s proactive approach to novel drug delivery technologies, including its support for fast-track and breakthrough designations in appropriate cases. Europe follows closely, driven by its emphasis on patient safety and innovation, especially in therapeutic areas like wound care and chronic pain management.
Asia-Pacific is emerging as a strategic growth hub for hydrogel-based drug delivery systems, largely due to rapid healthcare modernization, rising chronic disease prevalence, and cost advantages in R&D and manufacturing. Governments in countries such as China, India, and South Korea are actively investing in biotechnology infrastructure and public-private partnerships, further accelerating the development and commercialization of advanced drug delivery technologies.
However, the current market scenario also includes notable challenges. Despite growing interest, the commercialization of hydrogel-based products still faces hurdles related to regulatory complexity, scalability, and cost-effectiveness. Manufacturing high-quality hydrogel formulations requires advanced techniques and stringent quality control, which can inflate development costs. In addition, limited awareness among physicians and patients regarding the benefits of hydrogel delivery systems may hinder adoption in certain markets.
Another strategic concern is the need for long-term clinical evidence. While many hydrogel systems show promising results in early-phase studies, real-world data on long-term safety, efficacy, and cost-benefit are still limited. This makes healthcare providers cautious, especially in high-stakes therapeutic areas like oncology or central nervous system disorders. Companies that proactively invest in post-marketing surveillance and outcome-based studies are likely to gain an edge in market acceptance and payer support.
On a strategic front, the scenario is also seeing a rise in integrated therapeutic solutions that combine hydrogel-based delivery with digital health tools, such as biosensors or remote monitoring platforms. These hybrid solutions align with broader trends in personalized medicine and patient-centered care, offering a compelling value proposition for both providers and payers.
In conclusion, the current hydrogel-based drug delivery market scenario is one of active growth, increasing validation, and expanding opportunity. While challenges remain, the market is transitioning from experimental to mainstream status, driven by both innovation and strategic alignment across the pharmaceutical ecosystem. Companies that understand and adapt to this evolving scenario—through investment, collaboration, and patient-focused innovation—stand to secure a strong competitive position in the years ahead.