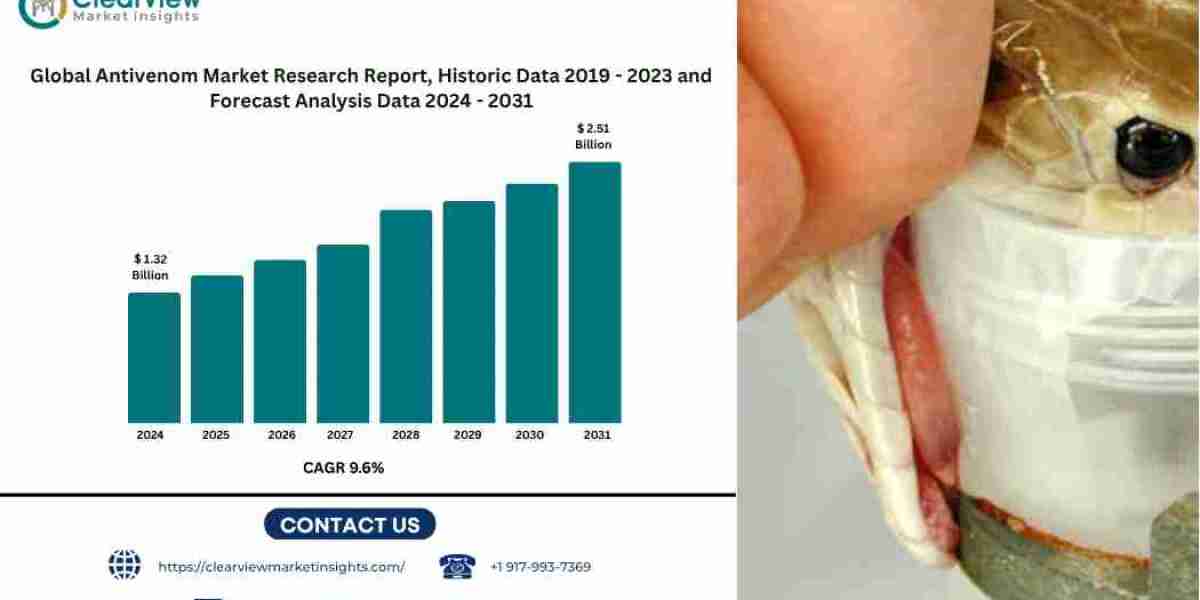
[Geneva, 5th, June 2025] – Clearview Market Insights (CVMI) forecasts that the global antivenom market will grow from USD 1.32 billion in 2024 to USD 2.51 billion by 2031, representing a compound annual growth rate (CAGR) of 9.6 percent. This expansion is driven by increasing demand from rural healthcare systems, WHO-backed prequalification efforts, and advancements in recombinant manufacturing.
"Antivenoms are no longer viewed as reactive emergency products—they are strategic components of rural public health infrastructure,” said Dr. Naomi Bediako, senior global health analyst at CVMI. “What was once a fragmented market is now seeing global alignment through multilateral procurement and innovation hubs."
Request Sample @ https://clearviewmarketinsights.com/report-details/global-antivenom-market/
Key Numbers
- 2024 Market Value: USD 1.32 billion
- 2031 Market Value: USD 2.51 billion
- Seven-Year CAGR: 9.6 percent
- Polyvalent Antivenoms Share (2024): 64%
- Asia-Pacific Market Share: 42%
Market Drivers
- High rural bite incidence in Asia, Africa, and Latin America
- Donor procurement programs led by WHO and UNICEF
- Next-generation freeze-dried and recombinant formats
- Rising government investment in emergency biologics
Company Highlights
- Instituto Clodomiro Picado scaled freeze-dried lines to meet Latin American surge.
- VINS Bioproducts initiated trials for polyvalent recombinant formulations.
- Bharat Serums won African Union multi-country tender.
- MicroPharm received EU innovation funding for venom-neutralizing monoclonals.
Regional Insights
- Asia-Pacific: Most cases and highest absolute demand for polyvalent formats.
- Africa: WHO procurement accelerates access; Sub-Saharan zone prioritized.
- Latin America: Brazil and Costa Rica dominate local production.
- North America & Europe: Innovation and orphan-drug approval zones.
2024–2025 Milestones
Quarter | Event | Outcome |
Q1 2024 | WHO prequalifies three new suppliers | Expands global access pipeline |
Q2 2024 | VINS launches recombinant trial | First-in-region polyvalent clinical trial begins |
Q3 2024 | African CDC launches pooled tender | Covers 15 countries for three years |
Q1 2025 | EMA grants orphan status to nanobody antivenom | Facilitates EU fast-track approval |
For more insights, visit https://clearviewmarketinsights.com/
About Clearview Market Insights
Clearview Market Insights is a global provider of healthcare and biologics market intelligence. We deliver actionable insights through real-world data, in-depth research, and a commitment to health equity and market transparency.
Media contact:
Bhavani K
Marketing and Sales Head
ClearView Market Insights
Mail: sales@clearviewmarketinsights.com
Phone: +1 917-993-7369