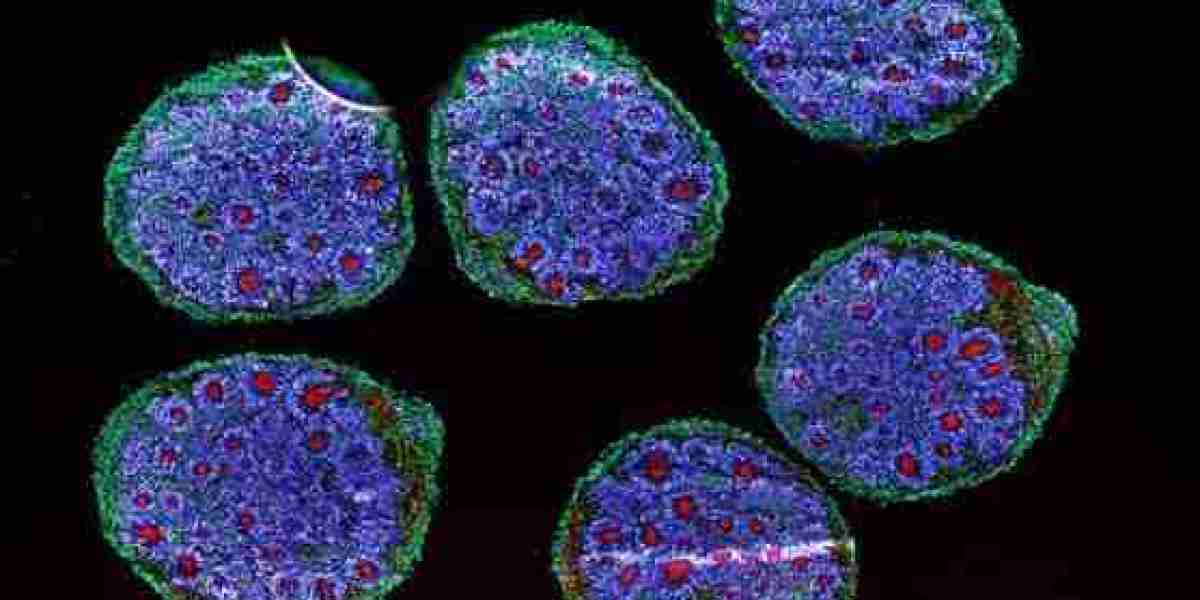
The organoids market growth is being significantly propelled by the pharmaceutical industry’s urgent need for more reliable and ethical alternatives to animal testing. Organoids—three-dimensional, stem cell-derived mini-organs—offer human-like models that better replicate human physiology, driving their adoption as superior tools in drug discovery and safety evaluation.
The Limitations of Animal Testing
For decades, animal testing has been a cornerstone of pharmaceutical research. However, it presents critical limitations: physiological differences between animals and humans often lead to inaccurate predictions of drug efficacy and toxicity. This discrepancy contributes to high failure rates in clinical trials, costing the industry billions annually.
Moreover, ethical concerns over animal welfare and increasing regulatory pressures to reduce animal use are prompting pharmaceutical companies to seek innovative alternatives. This shift is a primary growth driver for organoid technology.
Advantages of Organoids as Human-Like Models
Organoids are derived from human stem cells and self-organize into complex, tissue-like structures that replicate key aspects of real human organs. This biological relevance provides several advantages:
Improved Predictive Accuracy: Organoids better mimic human responses, enabling more precise assessments of drug effects, metabolism, and toxicity compared to traditional 2D cultures or animal models.
Personalized Medicine Potential: Patient-specific organoids allow tailored drug screening, enhancing treatment outcomes and reducing adverse effects.
Ethical and Regulatory Compliance: Organoids reduce reliance on animal testing, aligning with evolving regulatory guidelines and societal expectations for humane research.
Driving Market Adoption in Pharmaceuticals
Pharmaceutical companies are increasingly integrating organoids into their preclinical pipelines, motivated by the potential to reduce costly late-stage failures. Organoids are being applied in various domains such as oncology, neurology, and infectious diseases to:
Screen novel compounds with higher confidence
Identify off-target toxicities earlier
Study disease mechanisms in a physiologically relevant context
This adoption is facilitated by advancements in organoid culture techniques, automation, and data analytics, which improve scalability and reproducibility.
Supportive Regulatory Landscape
Regulatory agencies worldwide are recognizing the value of human-relevant models and encouraging their use to reduce animal testing. Guidelines promoting alternative testing methods and validation frameworks for organoids are increasing industry confidence and investment.
These supportive policies act as catalysts, accelerating the integration of organoid models into drug development workflows.
Broader Implications for Market Growth
Beyond pharmaceuticals, the shift towards organoids impacts cosmetics testing, environmental toxicology, and academic research, expanding market potential. The demand for more accurate, ethical, and cost-effective models is a common theme driving growth.
As awareness and technological maturity increase, the organoids market is projected to sustain robust growth rates through 2025 and beyond.
Conclusion
The organoids market growth drivers are deeply rooted in the pharmaceutical industry’s quest to replace animal testing with human-like, predictive models. Organoids offer a transformative solution that enhances drug discovery accuracy, addresses ethical concerns, and aligns with evolving regulatory expectations, making them key catalysts for market expansion.