The clinical trials market plays a pivotal role in advancing medical research, contributing significantly to the development of new drugs, therapies, and medical devices. These trials are essential for ensuring the safety, efficacy, and market readiness of medical innovations. Clinical trials are a critical phase in the drug development pipeline, making the market integral to pharmaceutical and biotechnology industries. The global clinical trials market is projected to grow substantially, driven by factors such as increased pharmaceutical R&D investments, the growing complexity of research, and outsourcing to Contract Research Organizations (CROs).
Market Size and Share
In 2024, the clinical trials market was valued at USD 51.15 billion. The market is expected to expand rapidly, with a compound annual growth rate (CAGR) of 8.50% from 2025 to 2034, which will see the market size reach approximately USD 115.65 billion by the end of the forecast period. The rising demand for more complex clinical research, especially with the increasing focus on biologics and biosimilars, is a significant factor contributing to the growth of the market. The expansion of the pharmaceutical R&D pipeline and advancements in clinical trial management technologies further bolster this market's growth trajectory.
Market Trends
Outsourcing of R&D Functions to CROs
As clinical trials become more complex, pharmaceutical companies are increasingly outsourcing research and development (R&D) functions to Contract Research Organizations (CROs). CROs provide specialized services such as data management, patient recruitment, and advanced testing services. This outsourcing trend allows pharmaceutical companies to focus on their core strengths while benefiting from CROs’ infrastructure, expertise, and regulatory knowledge, thereby reducing costs and accelerating time to market.
The Growing Demand for Biologics and Biosimilars
Biologics and biosimilars, due to their complex nature, require more extensive clinical trials for development and approval. The growing demand for these therapies is driving the clinical trials market, as they require specialized testing, evaluation, and trials that often extend over several years. Companies in the pharmaceutical industry are investing heavily in biologics, which is resulting in a surge in the number of clinical trials specifically focused on biologic therapies.
Technological Advancements and Innovation in Clinical Trials
The clinical trials landscape is seeing a significant shift with the integration of new technologies such as Artificial Intelligence (AI) and machine learning. These technologies are being used to enhance clinical trial designs, improve patient recruitment strategies, and streamline data management. Technological innovations also aid in the efficient monitoring of patient safety, which in turn improves the overall reliability and speed of clinical trials, ensuring better outcomes.
Strategic Partnerships for Clinical Trial Efficiency
The trend of forming strategic partnerships between pharmaceutical companies, CROs, and technology firms is becoming increasingly common. These collaborations aim to improve clinical trial efficiency, reduce timelines, and enhance the overall success rate of trials. Partnerships that integrate technology for better patient recruitment, data management, and trial monitoring are transforming how clinical trials are conducted, reducing costs and enabling faster market entry for new therapies.
Get a Free Sample Report with Table of Contents
Market Analysis
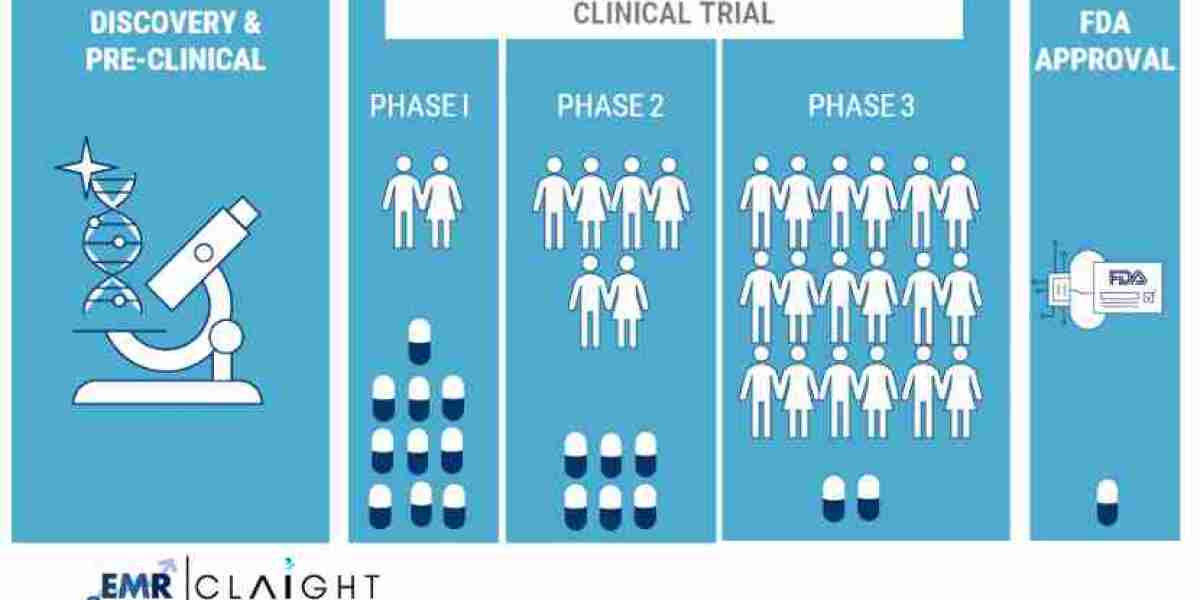
Phase Segmentation
The clinical trials market can be categorized into different phases, with Phase II and Phase III trials accounting for the largest share of the market. These phases typically focus on efficacy testing, optimal dosage determination, and safety monitoring. As the demand for complex biologics increases, these phases will likely expand to accommodate the rigorous testing required for such treatments. Phase IV trials, which monitor post-market safety, are also gaining traction as more drugs move toward commercialization.
Service Type Segmentation
Clinical trials encompass a wide range of services, with laboratory services, bioanalytical testing, and patient recruitment being some of the most vital components. Among these, decentralized clinical trials are gaining momentum as they provide patients with more flexibility and reduce the need for on-site visits. Clinical trial data management services are also becoming increasingly important, driven by the large volumes of data generated in clinical trials.
Therapeutic Area Segmentation
The clinical trials market is significantly impacted by therapeutic areas such as oncology, neurology, and infectious diseases. Oncology trials are among the most prevalent due to the rising global cancer burden, while neurology trials are increasingly focused on novel treatments for conditions like Alzheimer’s and Parkinson’s. Infectious diseases, especially after the COVID-19 pandemic, have also seen an uptick in clinical trials for vaccines and antiviral treatments.
Application Segmentation
Clinical trials serve various applications, including small molecules, monoclonal antibodies, vaccines, and cell & gene therapies. Small molecules remain dominant in the market, but the growing focus on gene therapies and vaccines has propelled new research efforts. Monoclonal antibodies, in particular, are critical in oncology and autoimmune disease treatments, leading to a surge in clinical trials dedicated to their development and testing.
Regional Insights
United States
The United States is the largest market for clinical trials, driven by its strong pharmaceutical and biotechnology sectors, advanced healthcare infrastructure, and a high level of R&D investment. The FDA’s rigorous regulatory framework ensures the safety and efficacy of new drugs, making the U.S. an attractive location for clinical trials. Additionally, the country's strong network of Contract Research Organizations (CROs) and healthcare providers offers a robust ecosystem for conducting diverse clinical trials.
Canada
Canada has become an increasingly prominent player in the global clinical trials market due to its high-quality healthcare system, favorable regulatory environment, and growing biotechnology industry. With a well-educated population and advanced research infrastructure, Canada offers a rich pool of clinical trial participants. The country's close ties with the U.S. also facilitate collaborations, enabling the seamless execution of cross-border clinical research projects.
Market Growth
The clinical trials market is expected to continue growing due to several key drivers. Pharmaceutical R&D investments are steadily increasing, with companies striving to meet the demand for new therapies, especially biologics. Additionally, the growing use of decentralized trials, aided by digital tools, is enabling more efficient and inclusive trials. Furthermore, the rise of personalized medicine and genomics will lead to a shift in how clinical trials are designed and executed. The market's future growth also hinges on innovations in data management and trial monitoring technologies, which will improve the speed and accuracy of trials.
Recent Developments & Challenges
- AI in Clinical Trials: Companies are increasingly adopting AI to streamline clinical trial processes, from patient recruitment to data analysis.
- Regulatory Changes: In response to the COVID-19 pandemic, many regulatory agencies, including the FDA and EMA, have introduced more flexible guidelines for clinical trials, particularly decentralized trials.
- Gene Therapy Advances: The growing success of gene therapies is likely to fuel new clinical trials, though their complexity remains a challenge for researchers.
- Patient-Centric Trials: There's a growing emphasis on making clinical trials more patient-centric, incorporating virtual visits and digital monitoring to improve recruitment and retention.
Key Players
IQVIA
IQVIA is a leading global provider of advanced analytics and contract research services, supporting pharmaceutical companies, biotech firms, and healthcare organizations with data-driven insights. Known for its expertise in health data management, IQVIA plays a critical role in shaping the clinical trials market by offering innovative solutions in trial design, patient recruitment, and monitoring. Their integration of artificial intelligence and machine learning technologies has enhanced the efficiency and accuracy of clinical trials.
Syneos Health
Syneos Health is a global biopharmaceutical solutions organization that offers a broad range of clinical trial services, including clinical operations, commercialization, and patient recruitment. The company’s expertise in both clinical and commercial solutions helps to optimize the drug development process. Syneos Health’s end-to-end capabilities ensure the rapid and efficient execution of clinical trials, making it a key player in the market.
WuXi AppTec
WuXi AppTec provides a broad range of services that support the entire lifecycle of drug development, from discovery through manufacturing and commercialization. With a strong presence in the clinical trials market, WuXi AppTec helps pharmaceutical and biotech companies reduce development timelines and improve trial outcomes. Their expertise spans preclinical testing, clinical trial management, and manufacturing services.
Laboratory Corporation of America Holdings (LabCorp)
LabCorp is one of the largest providers of laboratory services in the U.S. and plays a significant role in clinical trials by offering extensive diagnostic testing, clinical trial management, and bioanalytical services. LabCorp's broad network of clinical labs and its strong focus on data-driven insights make it a valuable partner for pharmaceutical companies conducting clinical trials.
Other key players in the market include Charles River Laboratories, Parexel International Corporation, Thermo Fisher Scientific Inc., ICON plc, Advanced Clinical, and Fortrea Inc.
FAQs
1. What is the clinical trials market?
The clinical trials market involves the research and development of new drugs and therapies through trials that test their safety, efficacy, and optimal use.
2. What factors are driving the growth of the clinical trials market?
Increased pharmaceutical R&D investments, growing demand for biologics and biosimilars, outsourcing to CROs, and technological advancements are the key drivers of market growth.
3. What are the main challenges in the clinical trials market?
Some challenges include regulatory hurdles, patient recruitment difficulties, and the growing complexity of trials, especially those involving biologics and gene therapies.
4. How is technology transforming clinical trials?
Technological innovations like AI and machine learning are improving clinical trial efficiency by optimizing trial design, data management, and patient recruitment processes.
5. Which regions are leading in the clinical trials market?
North America, particularly the United States, leads the global clinical trials market, followed by Europe and emerging markets in Asia-Pacific.