Introduction:
Duchenne Muscular Dystrophy (DMD) is a devastating genetic disorder that affects thousands of individuals, primarily young boys. It leads to progressive muscle degeneration and weakness. In recent years, the landscape of DMD research and treatment has witnessed remarkable advancements, particularly in the United States. This article delves into the latest trends and news in the DMD community, highlighting breakthroughs in genetic therapies, regulatory milestones, and the impact of patient advocacy groups.
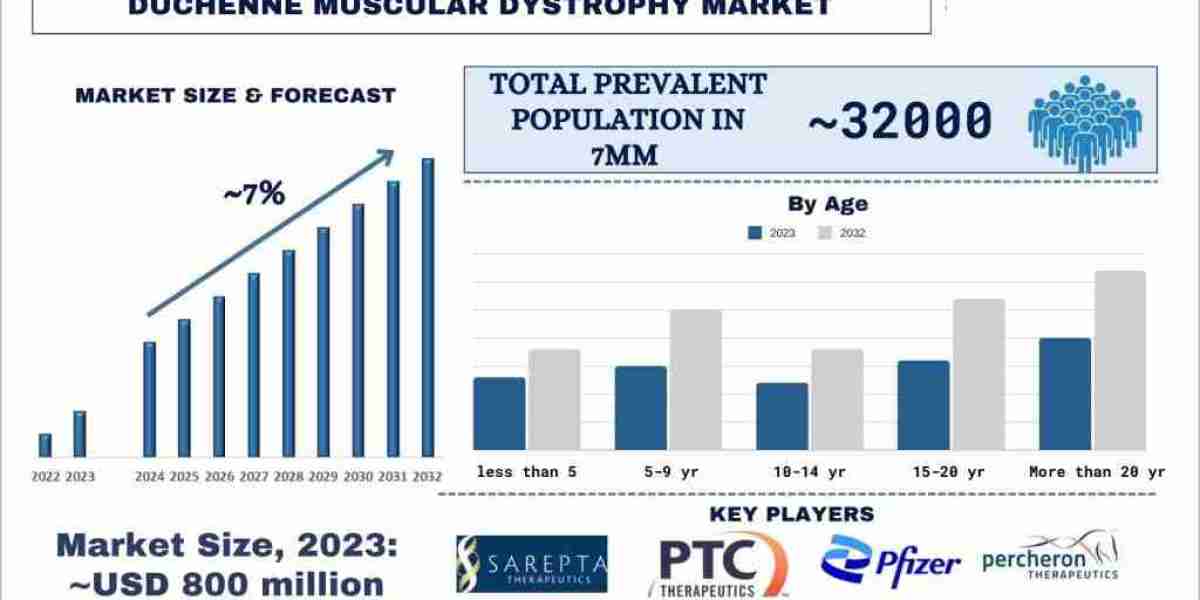
According to the UnivDatos Market Insights Analysis, advancements in genetic research, growing awareness campaigns, and advocacy efforts, regulatory agencies and pharmaceutical companies increasingly prioritize patient input in the drug development process, which will drive the 7MM scenario of the Duchenne Muscular Dystrophy Market. As per their “Duchenne Muscular Dystrophy Market” report, the 7MM market was valued at USD 800 Million in 2023, growing at a CAGR of about ~7% during the forecast period from 2024 - 2032 to reach USD XX billion by 2032.
Access sample report (including graphs, charts, and figures): https://univdatos.com/get-a-free-sample-form-php/?product_id=60948
Genetic Therapies: A New Horizon
One of the most promising areas in DMD treatment is the development of genetic therapies. These therapies aim to address the root cause of the disease by targeting the faulty gene responsible for producing dystrophin, a protein crucial for muscle health.
Exon-Skipping Therapies
Exon-skipping is a technique that uses synthetic compounds to skip over faulty parts of the dystrophin gene, allowing for the production of a functional, albeit shorter, version of the protein. Sarepta Therapeutics has been at the forefront of this innovation with its drug, eteplirsen (Exondys 51). Approved by the FDA in 2016, eteplirsen targets exon 51 and has shown promising results in stabilizing the disease's progression. Following eteplirsen, Sarepta has developed additional exon-skipping therapies targeting different exons, broadening the treatment scope for more patients.
Gene Editing and Therapy
Gene editing technologies, such as CRISPR-Cas9, have opened new avenues for potentially curing genetic disorders like DMD. In a landmark development, researchers at the University of Texas Southwestern Medical Center successfully used CRISPR to correct the dystrophin gene mutation in a mouse model of DMD. This breakthrough paves the way for human clinical trials, potentially providing a permanent solution to the disease.
Additionally, companies like Solid Biosciences and Pfizer are making strides in gene therapy, which involves delivering a functional copy of the dystrophin gene to muscle cells. Solid Biosciences' SGT-001 and Pfizer's PF-06939926 are among the leading candidates in this space, with both therapies showing encouraging early clinical trial results.
Regulatory Milestones and Support
The U.S. Food and Drug Administration (FDA) has played a crucial role in accelerating the development and approval of DMD therapies. The agency's commitment to supporting innovative treatments is evident through various regulatory designations and expedited approval pathways.
Orphan Drug Designation
Several DMD therapies have received orphan drug designation from the FDA, providing incentives such as tax credits for clinical testing, fee waivers, and market exclusivity upon approval. This designation is vital for encouraging pharmaceutical companies to invest in developing treatments for rare diseases like DMD.
Accelerated Approval Pathway
The FDA's accelerated approval pathway allows for the early approval of drugs based on surrogate endpoints that are reasonably likely to predict clinical benefit. This pathway has been instrumental in bringing DMD therapies to market more quickly, offering patients earlier access to potentially life-changing treatments. Eteplirsen, for example, was approved under this pathway based on its ability to increase dystrophin production.
Patient Advocacy: A Driving Force
Patient advocacy groups have been indispensable in driving DMD research and treatment progress. Organizations like Parent Project Muscular Dystrophy (PPMD) and the Muscular Dystrophy Association (MDA) have tirelessly worked to fund research, support clinical trials, and advocate for policies that benefit the DMD community.
Funding Research and Clinical Trials
PPMD and MDA have collectively raised millions to support research initiatives and clinical trials. Their efforts have helped bridge the funding gap, enabling researchers to explore innovative treatment approaches and bring them closer to clinical application.
Advocacy and Policy Change
Advocacy groups have successfully influenced policy changes that facilitate better access to treatments and care for DMD patients. For instance, PPMD's advocacy efforts were instrumental in securing the passage of the MD-CARE Act, which has significantly increased federal funding for muscular dystrophy research.
Real-World Impact and Future Directions
The advancements in genetic therapies, coupled with regulatory support and advocacy efforts, are transforming the DMD treatment landscape. However, the journey is far from over, and several challenges remain.
Access and Affordability
One of the significant challenges is ensuring that these advanced therapies are accessible and affordable for all patients. The high cost of genetic treatments poses a barrier, and there is a need for strategies to address pricing and reimbursement issues.
Long-Term Efficacy and Safety
While early results from clinical trials are promising, the long-term efficacy and safety of these treatments remain to be fully understood. Ongoing and future studies are crucial to monitoring patients over extended periods and gathering comprehensive data on the outcomes.
Related Reports-
Blood Pressure Cuffs Market: Current Analysis and Forecast (2024-2032)
Antibiotic Resistance Market: Current Analysis and Forecast (2024-2032)
Software as a Medical Device Market: Current Analysis and Forecast (2024-2032)
Opioid Use Disorder (OUD) Treatment Market: Current Analysis and Forecast (2024-2032)
Comprehensive Care
Beyond genetic therapies, comprehensive care involving multidisciplinary teams of healthcare providers is essential for managing DMD. This approach includes physical therapy, cardiac care, respiratory support, and nutritional guidance to improve quality of life and extend longevity.
Click here to view the Report Description & TOC- https://univdatos.com/report/duchenne-muscular-dystrophy-market/
Conclusion
The Duchenne Muscular Dystrophy landscape in the United States is witnessing a period of unprecedented innovation and hope. Advances in genetic therapies, robust regulatory support, and the unwavering dedication of patient advocacy groups are driving significant progress. While challenges persist, the collective efforts of researchers, clinicians, regulators, and advocates pave the way for a brighter future for those affected by DMD. As the field continues to evolve, the goal remains clear: to find a cure and improve the lives of all individuals living with Duchenne Muscular Dystrophy.